The Challenge: Finding special markers for risk prediction, prevention and management in twin pregnancies
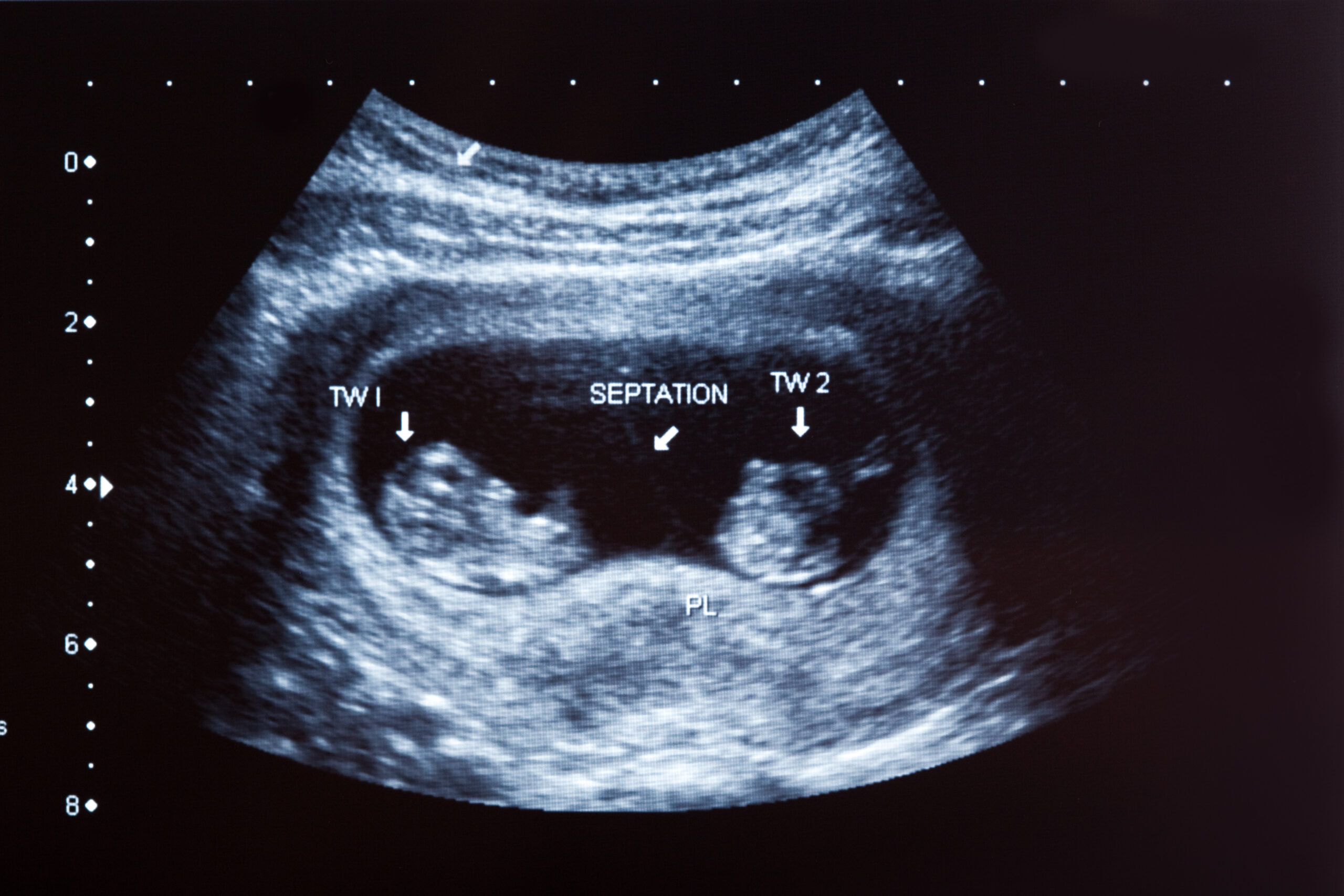
Twin pregnancies carry higher risks, yet standard prenatal care often doesn’t reflect their unique needs. The PRETWINSCREEN project developed new, tailored screening tools to improve early detection of complications and personalise care — helping clinicians better protect both mothers and babies.
Twin pregnancies are increasing worldwide due to assisted reproductive technologies such as in-vitro fertilization and later maternal age. However, twins face significantly higher risks than single pregnancies, including a greater likelihood of genetic abnormalities, preterm birth, gestational diabetes, and preeclampsia. Complications unique to twins, such as twin-to-twin transfusion syndrome (TTTS) in monochorionic twins, can have severe, lifelong consequences. Despite these risks, twins were often excluded from large-scale prenatal studies, leading to gaps in clinical knowledge and a lack of personalised screening tools.

The PRETWINSCREEN project, funded under ERA PerMed’s JTC2019, sought to bridge this gap by developing a multi-marker, personalised prenatal diagnostic model to predict and prevent complications in twin pregnancies. This ambitious initiative was coordinated by Prof. Ron Maymon (Itzhak Shamir Medical Center and Tel Aviv University, Israel), and brought together leading experts from five countries: Prof. Dr. Oliver Kagan (University of Tübingen, Germany), Prof. Annegret Geipel (University Hospital Bonn, Germany), Prof. Richard Brown (McGill University, Canada), Dr. Anna Goncé (BCNatal Hospital Clinic and IDIBAPS, Spain), and Prof. Kypros Nicolaides (King’s College Hospital and the Fetal Medicine Foundation, UK). This multinational partnership combined various clinical and methodological expertise, and diverse patient populations to address the unique challenges of twin pregnancies.
People are misled to think that twin is a simple duplicate of pregnancy with a single baby. They forget that each co-twin will become a unique personality, often having a different anatomical and genetic pool, and this starts already during the first months of the pregnancy. This study enabled us to shed light on the diversity of risk factors accounting for the increased rate of pregnancy complications in twin pregnancy and to develop methods of prediction, management and prevention of various complications either to each co-twin and/or to the pregnant women herself.
Prof. Maymon, coordinator of PRETWINSCREEN
Below is a summary of key PRETWINSCREEN studies, each addressing the unique complexities and risks of twin pregnancies — one of the most challenging areas in modern perinatal care.
A Global Collaboration for Better Outcomes
The international consortium built a comprehensive, evidence-based framework for managing twin pregnancies. The project followed 649 women and 1168 fetuses of which 24 were born as singletons since their other twin was either lost spontaneously or reduced due to a major genetic or structural abnormality. Women were enrolled from the first trimester of their pregnancy to birth, integrating data from clinical, biochemical, imaging, and genetic markers throughout. This large-scale, prospective, multi-center, multi-ethnic study strengthened the reliability and global applicability of the findings.
Key findings and innovations developed through PRETWINSCREEN include:
- Advanced risk prediction models for preeclampsia:
- First Trimester: PRETWINSCREEN developed a novel first-trimester prediction model for preeclampsia in twin pregnancies by analysing levels of cell-free fetal DNA (cffDNA), placental growth factor (PlGF), and maternal blood pressure (MAP). This model achieved a 90% detection rate at a 10% false positive rate, offering early intervention opportunities and improving pregnancy management. Unlike in singleton pregnancies, traditional markers like uterine artery blood flow (UtA-PI) were less effective in twins, emphasizing the need for tailored models. These findings were published in Prenatal Diagnosis.
- Making third trimester preeclampsia risk assessment in twin pregnancy accessible: Recognising the need for cost-effective diagnostics in resource-limited settings, the project developed a simplified third-trimester biochemical screening method for preeclampsia using just two biomarkers (sFLT-1 and PlGF). This approach is particularly beneficial for rural clinics and public health systems, ensuring wider access to prenatal risk assessment. This method is published in Fetal Diagnosis and Therapy (2025).
- A comprehensive three trimester model of preeclampsia prediction in twin pregnancies: Using machine learning, the PRETWINSCREEN consortium developed a comprehensive preeclampsia prediction model that incorporates data from all three trimesters. The study demonstrated that longitudinal testing improves predictive accuracy and revealed that having blood type B may be an additional risk factor in twin pregnancies. It also highlighted the need for tailored algorithms when predicting term versus preterm preeclampsia. Notably, the model enables clinicians to monitor the effectiveness of preventive treatments such as aspirin, supporting more personalised care. The results were presented at the 22nd Congress of Fetal Medicine in Prague (2025) and have been submitted for publication.
- Broader contributions to clinical practice and public health:
- Twin-specific growth charts: The project challenged the longstanding assumption that it is normal for twins to be smaller than singletons. By analysing growth trajectories, PRETWINSCREEN showed that healthy twin growth closely matches that of singletons. These findings, recently published in Obstetrics and Gynecology Research, led to the development of new twin-specific growth charts to help clinicians more accurately identify and manage true growth restrictions in twins.
- Early detection of fetal structural malformations: PRETWINSCREEN implemented a structured screening protocol based on anatomical ultrasound scans performed during all three trimesters, specifically adapted for twin pregnancies. Results from this large, multi-center study showed that over 80% of fetal malformations could be detected during the first and second trimesters, with more than 40% identified as early as the first trimester. The third trimester added further value by revealing developmental concerns relevant for delivery planning and postnatal newborn development monitoring. This approach allowed for improved prenatal counseling, earlier medical intervention, and better planning for delivery and neonatal care. Importantly, a cost-benefit model demonstrated that implementing this protocol would add only about 2 NIS (~0.5 €) per pregnancy in Israel, underscoring its affordability and potential for integration into standard prenatal care, thus leading to a proposal for changing the protocol for anatomical scans in twin pregnancies in Israel and a publication of the proposal for fetal structural anomalies evaluation across all three trimesters in twin pregnancies in the Archives of Gynecology and Obstetrics.
- Enhanced Non-Invasive Prenatal Screening (NIPS): PRETWINSCREEN demonstrated that combining first-trimester ultrasound with cell-free fetal DNA (cffDNA) testing significantly improves early fetal gender identification and the detection of X-linked disorders in twin pregnancies. This integrated approach enables more accurate genetic counseling and earlier, better-informed decision-making for families and healthcare providers. The findings were published in BMC Pregnancy and Childbirth (2023), based on a prospective study of twin pregnancies.
- Public health impact during COVID-19 pandemic: The project was one of the first to demonstrate that pre-pregnancy COVID-19 vaccination provided stronger immunity and better protection against complications in twin pregnancies. These findings, published in Scientific Reports, directly supported updates to public health recommendations and helped shape clinical guidance on optimal maternal vaccination timing.
Engaging Pregnant Women: A Patient-Centered Approach
Beyond scientific advances, PRETWINSCREEN placed significant emphasis on patient engagement. Pregnant women participating in the study formed social media groups and WhatsApp communities, sharing experiences and supporting one another. Their positive feedback and active involvement strengthened trust in the research and contributed to better recruitment and collaboration between patients and the medical teams. This underscores the importance of patient engagement in shaping personalised medicine research.
The Future of Twin Pregnancy Care
The PRETWINSCREEN project has contributed significantly to advancing standards of prenatal care in twin pregnancies. The project’s findings are already being integrated into clinical practice—for example, Israel’s Maccabi Healthcare System is piloting the preeclampsia prediction model.
Looking ahead, the research team envisions further advancements, including:
- Expanding molecular diagnostics using DNA, RNA, and exosome markers for early complication detection.
- Leveraging artificial intelligence (AI) and machine learning to refine predictive models.
- Exploring remote monitoring technologies, wearable devices, and home ultrasound solutions for personalised maternal care.
The Role of ERA PerMed Funding
ERA PerMed funding played a crucial role in enabling this multi-national, interdisciplinary collaboration. The financial and logistical support allowed researchers to conduct a large-scale, high-impact study, making the results statistically robust and applicable across diverse populations.
By integrating cutting-edge science, international expertise, and patient involvement, PRETWINSCREEN is helping to reshape how twin pregnancies are monitored and managed, with the potential to reduce risks and improve outcomes for both mothers and babies.
Key Publications
Svirsky R, Sharabi-Nov A, Maymon R, Kugler N, Landau Rabbi M, Brown R, Rodriguez HP, Peltier L, Nicolaides K, Meiri H. Prediction of Preeclampsia in Twins Using First Trimester: cffDNA Fraction, PlGF, and MAP. Prenatal Diagnosis. 2025;45(8):968–978. DOI: 10.1002/pd.6820. PMID: 40396999
Walter A, Geipel A, Simonini C, Strizek B, Sharabi-Nov A, Kugler N, Svirsky N, Segal Y, Mor-Shalom M, Valinsky L, Nasar S, Abu Hamed R, Meiri H, Maymon R, Cuckle H. Third Trimester Screening for Preeclampsia in Twins: Comparative Performance of FMF Algorithm, Roche and Quidel Triages. Fetal Diagn Ther. 2025; DOI: 10.1159/000546981. PMID: 40720944
Hamutal Meiri, PhD, Nadav Kugler, MD, Nataly Sharon, MD, Ran Svirsky, MD, Richard Brown, MD, Heidy Portillo Rodriguez, MD, Anna Goncé, MD, PhD, Mar Bennasar, MD, PhD, Antoni Borrell, MD, PhD, Julia Ponce, MD, Annegret Geipel, MD, PhD, Adeline Walter, MD, Corinna Simonini, MD, Brigitta Strizek, MD, PhD, Elisa Bevilacqua, MD, PhD, Federica Romanzi, MD, Karl Oliver Kagan, MD, PhD, Tanja Lennartz, MSc, Armin Bauer, PhD, Ron Maymon, MD, Kypros H. Nicolaides, MD, Yoram Louzon, PhD and the Pre-Twin Screen Consortium. Fetal Growth Chart in Twins Consistent with The Fmf Growth Curve: Prospective Multicenter Study. Obstetrics and Gynecology Research. 8 (2025): 79-90. DOI: 10.26502/ogr0182
Svirsky R, Maymon R, Kugler N, Orenstein A, Sharon NZ, Sharabi-Nov A, Meiri H, Maslenko M, Brown R, Portillo-Rodriguez H, Goncé A, Bennasar M, Matas I, Geipel A, Walter A, Simonini C, Strizek B, Bevilacqua E, Torcia E, Kagan KO, Steiner J, Lennartz T, Bauer A, Nicolaides KH, Borrell A; PreTwin Screen Consortium. The Pre-Twin Screen Consortium proposal for fetal structural anomalies evaluation across all three trimesters in twin pregnancies. Arch Gynecol Obstet. 2025 May 5. DOI: 10.1007/s00404-025-08044-0
Ron Maymon, Nadav Kugler, Narina Gulian, Adi Orenstein, Hamutal Meiri, Ran Svirsky. A proposal for changing the protocol for anatomical scans in twin pregnancies in Israel following our experience in the prospective evaluation and clinical management of twin pregnancies. Harefuah. 2025;164(1):25-31. PMID: 39931016
Svirsky R, Sharabi-Nov A, Sagie A, Meiri H, Orenstein A, Kugler N, Maymon R. High sensitivity and specificity in fetal gender identification in the first trimester using ultrasound and Noninvasive Prenatal Screening (NIPS) in twin pregnancies: a prospective study. BMC Pregnancy and Childbirth. 2023; 23:812. https://doi.org/10.1186/s12884-023-06133-z
Svirsky R, Landau Rabbi M, Abu Hamad R, Sharabi-Nov A, Kugler N, Galoyan N, Zilberman-Sharon N, Meiri H, Maymon R, Korach O. Vaccination in twin pregnancies: comparison between immunization before conception and during pregnancy. Scientific Reports. 2024;14(1):10813. https://doi.org/10.1038/s41598-024-61504-6
Meiri H, Kugler N, Svirsky R, Kagan O, Brown RN, Miron M, Borrell A, Goncé A , Bennasar M , Geipel A, Strizek B , Syngeleki A , Nicolaides K , Cuckle H, Maymon R. Pre-Twin Screen – A Multi-Disciplinary Approach for a Personalized Prenatal Diagnostics and Care for Twin Pregnancies. Int J Womens Health Wellness 2020, 6(1):110. http:/doi.org/10.23937/2474-1353/1510110
This project was funded by ERA PerMed project Pre-Twin Screen (project JTC2019-61) to develop a multi-marker, personalised, prenatal diagnostics model to predict feto-maternal complications in twin pregnancies.
Additional funding was provided by national institutions, including:
- Israel Ministry of Health Project #16874 (R.M., H.M.),
- Bundesministerium für Bildung und Forschung (BMBF) which was DLR Deutsches Zentrum für Luft- und Raumfahrt, # 01KU2001 (A.G.) ;
- Deutsches Zentrum für Luft- und Raumfahrt (DLR) (#01KU2001 section A (O.K.) and Section B (A.G.));
- Canadian Institutes of Health Research (CIHR) (ENP-168103) and Fonds de Recherche Santé Québec (FRSQ #294417);
- Departament de Salut, Generalitat de Catalunya, Spain (Expedient: SLD001/19/000002).
Ethics: Ethics approval was obtained from Shamir Medical Center and subsequently from all other participating centers. All participants provided written informed consent. The protocol was registered in Clinicaltrial.gov (ID: NCT04595214).

