Advancing Collaborative Implementation of Personalised Medicine Approaches in Healthcare

The European Partnership for Personalised Medicine, EP PerMed supported by the European Union under Horizon Europe, Grant Agreement N° 101137129, is pleased to launch its first Twinning Call 2026 for proposals on “Advancing Collaborative Implementation of Personalised Medicine Approaches in Healthcare”.
The Twinning Call 2026 is designed to accelerate the implementation of personalised medicine (PM) solutions or approaches from one country to another through peer-to-peer exchanges.
This call offers up to 50,000 EUR to activities that facilitate the exchange of solutions or approaches in personalised medicine and build up the capacities and capabilities of one or both participating parties. Twinning partnerships should last between 6 and 12 months.
Indicative Call Timeline
| Date | Event |
|---|---|
| 13 October 2025 – 26 February 2026 | Online matchmaking period |
| 10 December 2025 | Opening of the Call for Proposals submission |
| 17 December 2025 at 12:00 CET | Call information webinar |
| 15 January 2026 | Online matchmaking event |
| 26 February 2026 at 16:00 CET | Deadline for proposals submissions |
| 27 February – 20 March 2026 | Eligibility check |
| 23 March 2026 | Notification of eligibility check outcome and invite to online pitching sessions |
| 14 – 21 April 2026 | Online pitching sessions |
| 06 May 2026 | Final Results notification |
| 06 May – 08 June | Stand still period |
| Week beginning 08 June 2026 | Kick off meeting Onboarding and contracting begins |
Online Webinar and Call Contact Information
Matchmaking Platform – opening on Monday 13 October, closing on Thursday 26 February
Join the dedicated Matchmaking platform to list your organisation as a Twinning Donor or Twinning Receiver and find twinning partners.
Early Matchmaking Phase Webinar
Thursday 30 October 2025, 12.00 to 13.00 CEST
During the webinar, the EP PerMed team presented the objectives, eligibility criteria, and application process for the upcoming Twinning Call 2026 with a special focus on the ongoing early Matchmaking phase. Participants had the opportunity to ask questions and clarify any aspects before preparing their proposals.
Call information Webinar
Wednesday 17 December 2025, 12.00 to 13.00 CET
Register here to join an online webinar to hear more about the call and ask questions.
Online matchmaking event
15 January 2026, 12.00 to 14.00 CET
Register here to join an online matchmaking event to help you find twinning partners. Interested Twinning Donor and Receiver organisations will present their successful PM approaches or PM needs in the form of a pitch to further facilitate the Twinning partnership matchmaking process.
For speakers interested to pitch their PM solution or need: Please complete this form to express your interest to pitch.
For further information:
Please contact: calls_eppermed@eithealth.eu. When emailing, please pre-fix the email subject with “EP PerMed_Twinning”. This will ensure the right people pick up your email.
Aims of the call
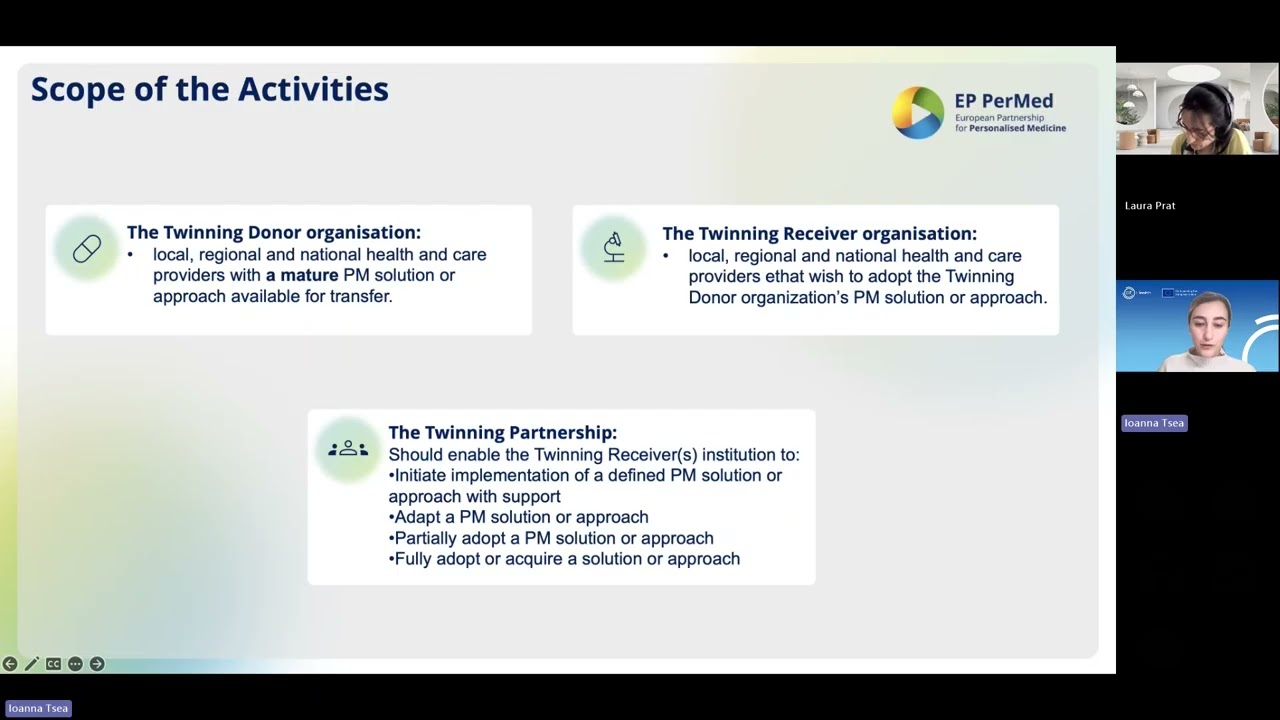
The Twinning Partnerships are aimed at local, regional and national health and care providers legally registered in a participating Horizon Europe country that wish to adopt PM solutions that are transferable from one provider to another. The Twinning Call will pair at least two and up to three institutions together. One institution/organisation/company will be the Donor, and the other up to two institution(s) will be the Receiver(s). The Donor institution is responsible for sharing its experience, knowledge, and practices relevant to the specific PM solution or approach with the Receiver organisation(s).
Twinning partnerships should enable the Twinning Receiver(s) institution to:
- Initiate implementation of a defined PM solution or approach with support
- Adapt a PM solution or approach
- Partially adopt a PM solution or approach
- Fully adopt or acquire a solution or approach
Activities must demonstrate their link to PM and may cover the whole health value chain: diagnosis, theranostics, prevention strategies, screening, early detection, and diagnostic tools, new personalised treatments, interventions and patient management, recovery and follow-up support systems. The proposed twinning concepts must have a clear direct or indirect impact on patient care.
Examples of what could be exchanged may cover:
- Implementation of a specific care pathway in PM or defined PM approach within the Twinning Receiver’s organisation, following a model that has already been effectively established by the Twinning Donor in its own organisation.
- Implementation of a specific technological solution in PM within the Twinning Receiver’s organisation, based on a PM solution that has already been successfully deployed by the Twinning Donor in its own organisation.
This Call will not award funding to projects that aim at exchanging knowledge about what PM is in general or for pure research purposes. PM solutions and twinning concepts proposed must have a clear direct or indirect impact on patient care.
Activities that will not be funded and are out of scope include:
- Any technological solution that is below Technology Readiness Level (TRL) 9. TRL 9 is the top of the TRL scale and signifies that a technology has been successfully tested in an operational environment and is ready for full deployment. At TRL 9 the solution has undergone extensive validation and demonstrated its functionality in real-world conditions, is no longer a prototype but a fully developed and commercially viable product.
- Policy level interventions.
- Regulatory or ethical policies or guidelines.
- Public awareness campaigns.
- Preliminary groundwork that does not progress into initial execution phases.
- Twinning partnerships that are mainly profit-oriented, mainly for marketing purposes of a commercially available product or only aim at exchanging information about PM or PM approaches, with no prospects of PM implementation in health and care institutions.
Eligibility criteria
General Conditions
Applications must meet all the below eligibility criteria to be invited to the independent expert evaluation stage. Each application will be reviewed for these eligibility criteria by the Call Secretariat – EIT Health, and members of the EP PerMed consortium.
- Applicants must submit their proposal via the application platform before the deadline on 26 February 2026 at 16:00 CET.
- Applications must be written in English.
- The Twinning language is English, and the project grant agreement (PGA) must be signed between EP PerMed and all parties in the application consortium in its English version.
- Applications must be submitted in English.
- Twinning activities must be carried out in English.
- Applications can request a maximum of 50,000 EUR of funding including overheads.
- Applicants must use and upload any templates requested and provided in the application platform. Applications that upload the incorrect template or a modified version of the template will be considered ineligible.
- To be eligible for funding, all applicants must be legally registered in a Horizon Europe country or associated country.
- The PM solution or approach must be mature for implementation. In the case of technological solutions this means TRL 9 and CE-marked if required.
- Application must meet the definition of PM as defined in this call.
- Partners (Beneficiaries/Affiliated Entities/Associated Partners) within the EP PerMed consortium are not eligible for this Call.
- Individuals are not eligible for this Call.
Consortium Eligibility
Applicants are encouraged to use the dedicated Matchmaking platform to fulfil the consortium composition requirements. A dedicated online matchmaking event on 15 January 2025 will also be held for interested applicants.
The following conditions apply to the composition of consortia:
- A consortium submitting an application must include one Donor organisation, and at least one, but not more than two Receiver organisations.
- The consortium must be transnational, involving a minimum of two and a maximum of three organisations legally registered in at least two different EU Member State countries or countries associated to Horizon Europe.
- Consortium members must be legally and financially independent of each other.
- Proposals must be submitted by applicants belonging to one of the following categories:
- Twinning Donor organisations: local, regional and national health and care providers established in an EU Member State or country associated with Horizon Europe with a PM solution or approach available for transfer.
- Twinning Receiver organisations: local, regional and national health and care providers established in an EU Member State or country associated with Horizon Europe that wish to adopt the Twinning Donor organisation’s PM solution or approach.
- The consortium must be completed at the time of submission.
Eligible costs and project duration
- The types of activities that may be awarded funding include hosting meetings or working groups, travel expenses, or fees for professional services.
- Costs for the further development of PM solutions are not eligible.
- Costs for devices, instruments and equipment are not eligible.
- Funding will support the Twinning activity up to the submission of the final activity report, which must be delivered no later than 6 May 2027.

